In the basic research and clinical translation of mesenchymal stem cells (MSCs), the preservation of cell viability, stability, and functional retention after cryopreservation and thawing serves as the core indicators determining experimental reproducibility and clinical safety of products. Today, leveraging rigorous controlled experiments conducted by clinical-grade enterprises, we provide a comprehensive interpretation of the core performance characteristics of CS-SC-D1 cryopreservation medium, offering valuable insights for partners in both scientific research and industrial applications.
Due to its pluripotent differentiation and immunomodulatory properties, MSCs are widely applied in regenerative medicine. However, they are highly sensitive to oxidative stress and ice crystal damage during cryopreservation, and traditional protocols generally face the following challenges:
Rapid activity decay: The activity level drops sharply after recovery, failing to meet the requirements for long-term downstream cultivation and formulation.
Clustered apoptosis: Ice crystal damage leads to cell membrane rupture and cell adhesion, resulting in a significant decrease in effective recovery rate.
Loss of function: significant decline in proliferative, immunomodulatory, and paracrine functions (e.g., HGF secretion);
Insufficient stability: Significant variability in efficacy across different brands, making it challenging to adapt to standardized clinical-grade formulation production.
To address these challenges, CS-SC-D1 utilizes an innovative formulation and core technology to establish a comprehensive cryopreservation protection system, with five key advantages that directly meet the demands:
cGMP manufactured : Utilizing excipients compliant with the full pharmacopoeia, strictly adhering to GMP production standards, with precise limits on chemical composition, and designed without serum, protein, or animal-derived components, completely avoiding xenogenic contamination and safety risks, fully meeting clinical-grade compliance requirements.
Dual mechanism of ice control and anti-apoptosis: inhibits ice crystal formation and oxidative stress, reduces cell membrane damage, decreases apoptosis rate, while supporting high-density cryopreservation of MSCs to meet large-scale production requirements;
Oligomeric Cluster-Exclusive Design: Incorporates dispersed protective components to effectively reduce post-thaw cell aggregation rates, enhance effective recovery rates, and meet the demands of high-density cryopreservation recovery.
Complete functional preservation: Maximizes the maintenance of MSC activity, immunomodulatory and paracrine functions, while accommodating cryopreservation of various MSC types including umbilical cord and bone marrow MSCs, as well as hematopoietic stem cells and iPSCs.
Ready-to-use + simplified operation: No prior preparation required, ready-to-use upon opening, supports direct freezing at-80°C without programmed cooling, reduces equipment dependency and operational complexity, significantly enhances experimental and production efficiency, and has completed NMPA registration for enhanced compliance assurance.
This experiment was conducted by a clinical-grade enterprise specializing in MSC drug development. Using fresh human umbilical cord mesenchymal stem cells (hUC-MSC) as the control group, the study evaluated the performance of three brands of cryopreservation solutions under cryopreservation conditions with a density of 2×10^7/mL, through multidimensional validation.
Survival rate performance: In the CS-SC-D1 group, the survival rate remained stable above 95% at 8 hours post-recovery, showing no significant difference from fresh cells .
Clustering rate performance: The CS-SC-D1 group maintained a stable clustering rate of 20%-25%, significantly lower than the competitors, effectively reducing downstream processing interference.
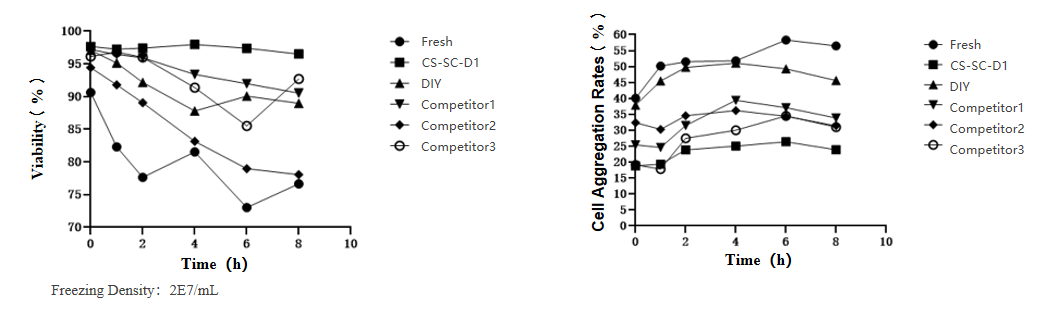
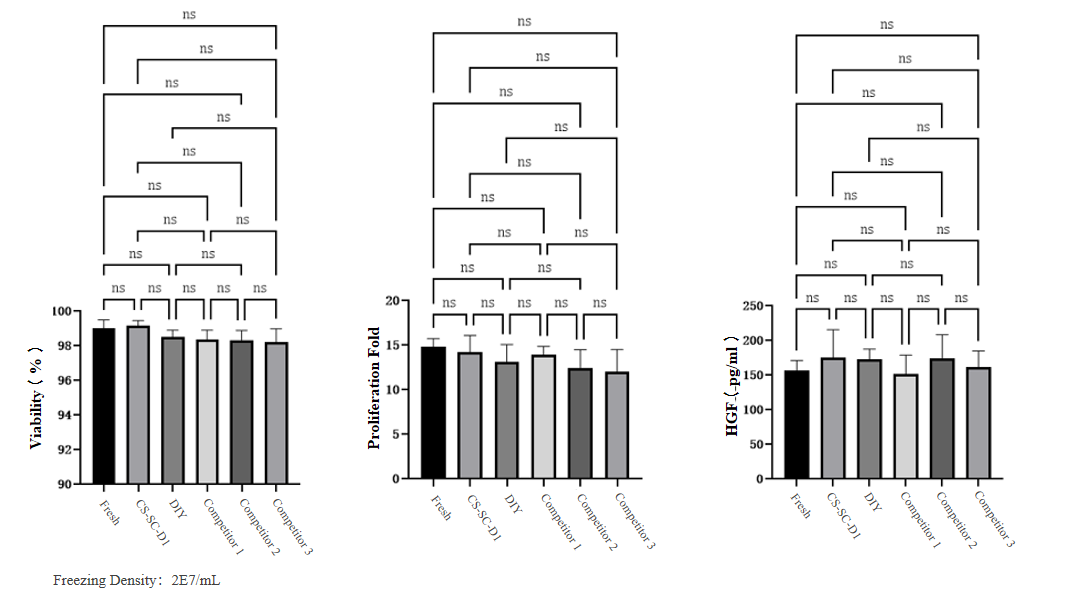
Recovery rate: The recovery rate of CS-SC-D1 group was>98%, showing no statistically significant difference from fresh samples.
Proliferation fold: The proliferation rate was comparable to that of fresh cells.
HGF secretion: The CS-SC-D1 group showed no difference in secretion levels compared to fresh cells.
This leading MSC drug development company achieved the following outcomes after replacing with CS-SC-D1 without modifying operational procedures: stabilized viability rate>98% and 60% reduction in cell aggregation rate, thereby accelerating the IND application process.
Research institution: Conducts long-term studies and maintains cell banks to ensure reliable experimental data;
Cell therapy company: Large-scale production of clinical-grade MSC preparations, achieving standardized compliance;
Medical institutions: Long-term preservation and translational application of MSC clinical samples;
CRO companies: Establish standardized efficacy evaluation models and provide stable experimental platforms.
CS-SC-D1, supported by measured data, breaks through the limitations of traditional cryopreservation protocols and provides stable impetus for MSC research and clinical translation. For complete experimental reports or sample trials, please leave a message for consultation.